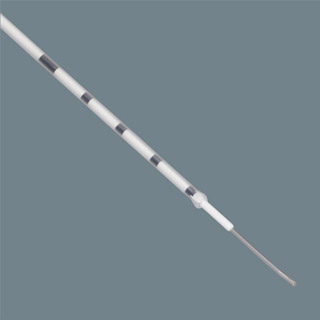
The Guardia Pro Protective Embryo Transfer is noted to be the latest addition to Cook Medical’s large range of products for Assisted Reproductive Technology (A.R.T.). Evidently, this uniquely engineered transfer catheter even comprises of the company’s Microvol technology, which is presumed to aid in reducing the fluid volume needed for embryo transfer. This reduction is in turn believed to be helpful in lowering the chances of embryo migration, along with increasing the precision of embryo placement.
“The launch of the Guardia Pro is an important step forward for the industry and for those undergoing IVF. Our unique product creates a greater opportunity for successful embryo implantation. We are committed to simplifying procedures for patients and clinicians and with this device we hope to improve the process of embryo transfer,†says Christina Anná, Vice President of Cook Medical’s women’s health strategic business unit.
Coming to the structure of this latest product, its outer sheath notably shields the embryos through the entry. After which it goes on to the next level and opens in petals to ensure the accurate and gentle placement of the embryo in the uterine cavity. Notably, this product is stated to be intended for one-time usage.
It was further also declared that this innovative transfer catheter may simplify the process of embryo placement and also be instrumental in reducing the amount of discomfort felt by the patients undergoing this treatment.
Guardia Pro Protective Embryo Transfer Catheter has notably been introduced at the European Society of Human Reproduction and Embryology (ESHRE).
