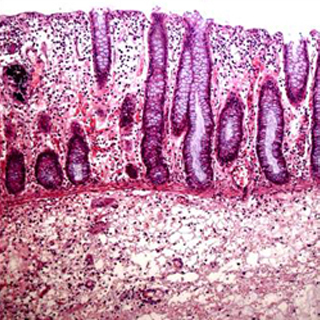
Inflammatory bowel disease (IBD) is a collection of inflammatory conditions of the colon and small intestine.
Dr Erik Glocker (UCL Infection & Immunity) apparently discovered the first mutation in the protein IL10R2 recognized in the research. Examination of supplementary patients with premature onset colitis supposedly exposed mutations in IL10R1.
Dr. Glocker commented, “This discovery is a milestone in research on inflammatory bowel disease, and will enable us to gain further insights into the physiology and immunity of the intestine.â€
IL10 apparently plays a vital function in monitoring the body’s inflammatory responses. The human body is said to be incessantly producing local inflammatory responses to manage microbial infections and to mend injury from toxins. But if that inflammatory response is not correctly controlled, tissues could be terribly damaged.
When either IL10R1 or IL10R2 is mutated, the warning signs from IL10 cannot be received, and the ensuing inflammation apparently causes tissue damage, in particularly the gastrointestinal system.
The gene identification effort headed by Professor Bodo Grimbacher at UCL Medical School (Royal Free Campus Hampstead), and leader of the Marie-Curie Excellence Research Group, mentioned, “This discovery will lead to future therapeutic options not only in children but potentially also in adult patients with IL10 signalling problems.â€
Other research groups concentrating chiefly on adult-onset IBD have apparently recognized dozens of genes and variants that influence the danger for IBD, but this is claimed to be the first research to illustrate that a single genetic mutation could be enough to cause the disease.
Finding the genetic mutations supposedly meant that the researchers were able to effectively treat one of the research patients with a bone marrow transplant (BMT). The patient, who had not reacted to other therapies, apparently illustrated an impressive development after the BMT and has supposedly remained in remission for more than a year.
The resolution to treat one of the research participants with a BMT was due to numerous issues. A prerequisite for considering a BMT is that the affected gene is thought to be generally active in bone-marrow derived cells, which is the case with both of the genes mutated in the patients. The disease is also believed to be acute in patients with the known mutations that the possible advantages of a BMT may overshadow the risks. The patient also apparently had a fit sibling who matched as a donor, which facilitated the transplant.
The joint research comprised of researchers from UCL, the National Center for Biotechnology at the National Institutes of Health, Hannover Medical School in Germany and various other institutions.
